Achieving Regulatory Compliance in Systems Engineering: Key Considerations
Ensuring compliance is paramount for systems engineering projects. According to MetricStream, a staggering $44.94 billion in fines were levied on businesses in the US and Canada alone in 2021 for non-compliance issues, highlighting the significant financial risks involved. Similar trends are seen globally, with organizations facing hefty penalties for breaches.
Just like established industry standards (or frameworks) ensure the safety of a bridge, regulatory compliance serves as a critical safeguard for your systems. By adhering to industry standards and legal requirements, you protect users from potential harm and your business from costly legal repercussions.

This blog will equip you with key considerations to navigate the path of regulatory compliance in systems engineering. We’ll explore strategies that can help you achieve both adherence to regulations and unhindered innovation.
What is Regulatory Compliance, and Why is it Necessary?
Regulatory compliance in product/service development constitutes the paramount adherence to a well-defined corpus of rules and principles established by governing bodies and industry associations. These regulations serve as mandatory edicts designed to ensure the safety, security, and efficacy of a diverse range of products and systems deployed within the marketplace.
Consider the established regulatory frameworks akin to the essential traffic laws that govern our roadways. While traffic laws may, at times, present logistical hurdles, their implementation is undeniably critical for ensuring the safety of all participants within the transportation system. Similarly, regulations in systems engineering serve a far more expansive purpose than simply safeguarding users and the environment. These regulations establish a comprehensive set of guiding principles that ensure the integrity, reliability, and responsible operation of the complex systems we create.
For instance, medical devices need to be rigorously tested to ensure they’re safe and effective for patients. Likewise, regulations for aviation systems exist to prevent catastrophic failures that could endanger lives. By complying with these regulations, you’re not just following the rules, you’re building trust and demonstrating a commitment to responsible innovation.
Let’s Understand the Regulatory Terrain
Regulatory standards vary widely across industries. Aerospace and healthcare group companies follow very strict standards, respectively of AS9100 (Aerospace) and ISO 13485 (Medical Devices). Within such or similar standards, several tangible benefits have been achieved, such as customer satisfaction rates, safety records, and working efficiency that other organizations under different standards can only make comparisons with. In 2021, the International Organisation for Standardisation issued more than 1.5 million ISO certifications worldwide, demonstrating the global emphasis on quality and safety compliance standards in various sectors (ISO, 2021).
New technologies and creative approaches are constantly pushing the boundaries of what’s possible in the world of systems engineering. However, to ensure this innovation translates into responsible development, Systems engineers must familiarize themselves with industry-specific regulations and regulatory bodies that impact their field, as well as the specific standards required. Common standards include:
- ● FDA (Food and Drug Administration) – United States agency that oversees the safety of food, drugs, and medical devices.
- ● GDPR (General Data Protection Regulation) – European Union regulation that sets standards for data protection and privacy for individuals within the EU.
- ● ISO (International Organization for Standardization) – United States agency that oversees the safety of food, drugs, and medical devices.
- ● NIST (National Institute of Standards and Technology) – U.S. agency providing cybersecurity frameworks and standards for federal information systems.
- ● EMA (European Medicines Agency) – European Union body responsible for the evaluation and supervision of medicinal products.
- ● OJK (Otoritas Jasa Keuangan) – Financial Services Authority of Indonesia that regulates and supervises the financial services sector in Indonesia.
- ● FCA (Financial Conduct Authority) – UK regulatory body overseeing financial markets and ensuring consumer protection in the financial industry.
- ● SEC (Securities and Exchange Commission) – U.S. agency that regulates securities markets, protecting investors and maintaining market integrity.
- ● APRA (Australian Prudential Regulation Authority) – Australian regulator responsible for ensuring the stability of the financial system, covering banks, insurers, and superannuation funds.
- ● PCAOB (Public Company Accounting Oversight Board) – U.S. organization overseeing audits of public companies to protect investors and promote public interest in audit reports.
- ● RBI (Reserve Bank of India) – India’s central bank that manages monetary policy and regulates banking operations.
- ● FINMA (Swiss Financial Market Supervisory Authority) – Swiss regulatory body supervising banks, insurance companies, and financial markets in Switzerland.
- ● CSA (Canadian Standards Association) – Canadian organization that develops standards for health and safety across various sectors.
- ● MAS (Monetary Authority of Singapore) – Singapore’s financial regulatory authority overseeing banking, insurance, securities, and currency issuance
- ● ACMA (Australian Communications and Media Authority) – Regulates communications and media services in Australia to safeguard public interests
- ● CNIL (Commission Nationale de l’Informatique et des Libertés) – France’s data protection authority, ensuring data privacy and regulatory compliance with GDPR.
- ● HKMA (Hong Kong Monetary Authority) – Hong Kong’s central banking institution that manages monetary policy and oversees financial stability.
- ● BaFin (Federal Financial Supervisory Authority) – German regulatory agency responsible for overseeing financial institutions and protecting consumer interests.
- ● CFDA (China Food and Drug Administration) – Chinese authority responsible for regulating food, drugs, and medical devices to ensure public health and safety.
- ● JFTC (Japan Fair Trade Commission) – Japan’s antitrust regulator, ensuring fair trade practices and competition within the Japanese market.
These standards are non-negotiable for companies operating in regulated environments, particularly if they handle sensitive data or work in highly scrutinized industries like finance and healthcare. The specific regulations applicable to your system depend on several key factors:
- ● Industry: Regulations vary significantly across industries. Understanding the regulations specific to your client’s domain is crucial.
- ● Location: Geographical location plays a role in determining the relevant regulatory framework.
- ● System Function: The system’s intended use can influence applicable regulations. For example, systems collecting personal data might need to comply with data privacy regulations like GDPR.
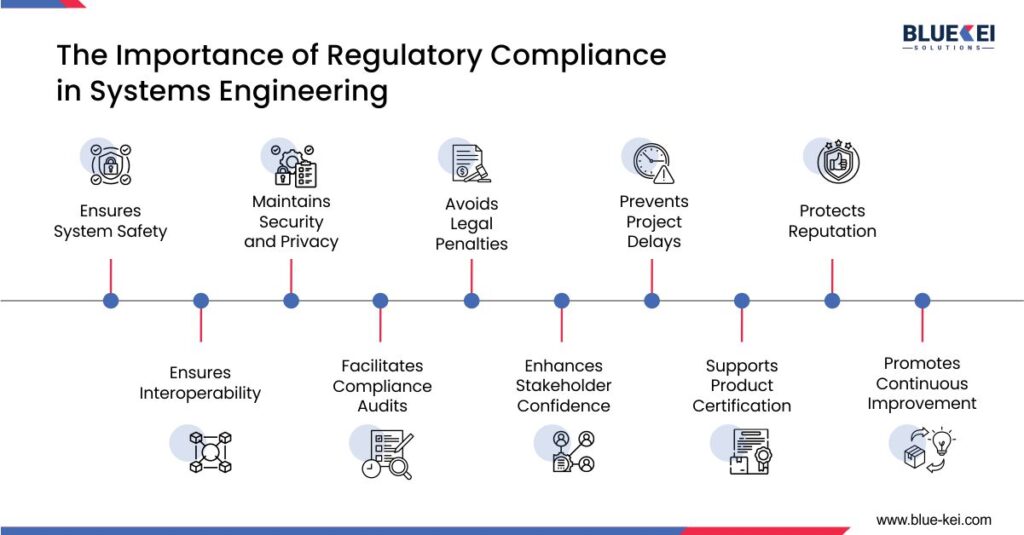
Key Considerations in Achieving Compliance in Systems Engineering Projects
- Building Compliance into the Systems Development Lifecycle (SDLC)
Integrating compliance checks from the start of the systems development lifecycle (SDLC) ensures that potential issues are addressed early. This approach, often termed “compliance by design,” helps avoid costly rework and keeps projects on track.
2. Cyber security as a Regulatory Mandat
With the increasing threat of cyberattacks, cybersecurity regulations are becoming stricter. Systems engineering projects must now incorporate cybersecurity standards to protect sensitive data and maintain system integrity. Many regulatory standards, like NIST’s Cybersecurity Framework and ISO 27001, require systems engineers to implement protocols for data protection, incident response, and regular audits.
The average cost of a data breach in 2024 is $4.88 million, as per an IBM report, which 10% higher than the last year, highlighting the necessity of cybersecurity in compliance.
3. Automation and Compliance Management Tools
Today, compliance is often supported by technology. Automated compliance tools help systems engineers track requirements, generate reports, and ensure consistent adherence to standards throughout the project lifecycle. Tools like IBM OpenPages and SAP GRC automate these tasks and provide real-time insights, simplifying the compliance process. The recent PwC’s analysis indicates that a 15% rise in automation may result in a 10% reduction in compliance costs for numerous companies currently.
4. Building Compliance into the Systems Development Lifecycle (SDLC)
Compliance isn’t a one-time task but an ongoing process. Regular audits and monitoring are essential to maintaining adherence to standards. They help identify potential issues before they escalate and ensure that all processes meet the latest regulatory standards.
Some Aspects in Data-Centric Compliance
Systems engineering often involves handling sensitive information. Here are some key considerations for ensuring data compliance:
- ● Partitioning Training Data for Data Privacy: Regulations like GDPR mandate responsible data handling practices. Partitioning training data for AI models can help mitigate privacy risks.
- ● Auditability for Data Annotations:Maintaining a clear audit trail for data annotations is crucial. This allows you to demonstrate the accuracy and integrity of your data throughout the development process.
- ● Data Compliance Throughout the Release Lifecycle:Data compliance is an ongoing process.
Ensure your system adheres to relevant regulations from the initial data collection stage through model deployment.
Building a Culture of “Compliance is Cool”
By fostering a culture of awareness within your team, you can make it a seamless part of the development process. This involves training team members on relevant regulations and best practices can be helpful. The more everyone understands the “why” behind compliance, the smoother the entire process will be-
Risk Management: Your Compliance Copilot
Imagine navigating a treacherous mountain pass without a map. That’s what building a system without a robust risk management plan feels like. Identify potential compliance pitfalls early on and have a plan B (or C, or D) ready. Tools like FMEA can be your best friend here, helping you pinpoint potential issues before they snowball.
Traceability: Leaving a Breadcrumb Trail
Remember Hansel and Gretel and their clever use of breadcrumbs? Traceability is your version of that. Document every design decision, test result, and any detours you take along the way. This clear audit trail will be your lifesaver when it comes to demonstrating compliance during those inevitable regulatory reviews.
Documentation Done Right: From Chaos to Clarity
Clear and well-organized documentation is essential for demonstrating compliance to regulatory bodies. This includes design specifications, test plans, risk assessments, and other relevant documents. An effective documentation management system ensures everything is readily accessible for audits and reviews, streamlining the compliance process.
Here are some key aspects of effective documentation management:
- ● Centralized Repository: Maintain a central repository for all compliance-related documents. This allows for easy access and version control.
- ● Standardized Templates: Utilize standardized templates for commonly used documents like test plans and risk assessments. This promotes consistency and clarity.
- ● Metadata and Search Functionality: Implement a system for tagging documents with relevant metadata and enabling efficient search functionality. This allows you to quickly locate specific information when needed.
By prioritizing well-organized documentation, you can navigate regulatory requirements with greater ease and efficiency.
Final Thoughts
Regulatory compliance forms the proactive approach to developing safe and reliable systems in systems engineering. Adopting best practices, exploiting compliance tools, and awareness of industry standards all reduce risks but increase trust with stakeholders as well. By following these key considerations, you can transform compliance from a headache into a launchpad for innovation. Remember, fostering a culture of compliance within your team and embracing a proactive approach are the keys to ensuring your systems meet the highest standards of safety and performance.
At BlueKei, we are experts in navigating the complexities of compliance while keeping your project on track. Contact us today and let’s discuss how we can help you turn your innovative ideas into reality!
If you are interested in understanding how to adopt systems engineering and model based systems engineering practices within your organization, reach out to BlueKei Solutions team at info@Blue-Kei.com. We specialize in systems engineering consulting, project executions, process adoptions such as compliance to ISO15288, ARP 4754A, ISO 42020, digital transformations. We can also conduct capability development workshops which are experiential and tailored to your needs. With systems engineering adoption you can address the complexity, manage evolving risks and bring transformation in communication within your organization through digitalization and create the digital thread.

